

Many of these polymers are used as solid materials and must be isolated from the aqueous dispersion after polymerization. When water-soluble polymers are used as stabilizers instead of soap, the repulsion between particles arises because these water-soluble polymers form a 'hairy layer' around a particle that repels other particles, because pushing particles together would involve compressing these chains.Įmulsion polymerization is used to make several commercially important polymers.
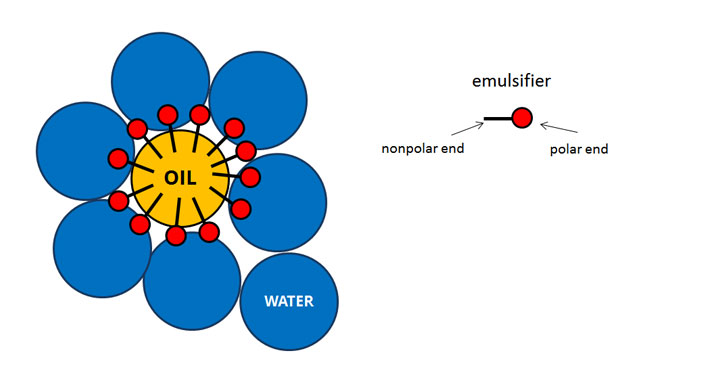
The particles are prevented from coagulating with each other because each particle is surrounded by the surfactant ('soap') the charge on the surfactant repels other particles electrostatically. These latex particles are typically 100 nm in size, and are made of many individual polymer chains. Rather than occurring in emulsion droplets, polymerization takes place in the latex/ colloid particles that form spontaneously in the first few minutes of the process. The name "emulsion polymerization" is a misnomer that arises from a historical misconception. Water-soluble polymers, such as certain polyvinyl alcohols or hydroxyethyl celluloses, can also be used to act as emulsifiers/stabilizers. The most common type of emulsion polymerization is an oil-in-water emulsion, in which droplets of monomer (the oil) are emulsified (with surfactants) in a continuous phase of water. As described above, phospholipid molecules can help form a “molecular cage” to allow for oil phases to be separate from water phases, as shown in the schematic below.Emulsion polymerization is a type of radical polymerization that usually starts with an emulsion incorporating water, monomer, and surfactant. This presence of a phospholipid stabilizes the emulsion, preventing the oil and water components from separating. In an emulsion, a phospholipid would surround the dispersed droplets, creating a micelle, forming a barrier between the water and oil. A common surfactant is a phospholipid, such as lecithin, which contains a polar headgroup that interacts with water, and a nonpolar tail that interacts with oils. Additionally, surfactants have the ability to form micelles, which are a type of molecular “cage” that forms around solute molecules that are normally immiscible in a particular solvent (ie nonpolar molecules can be protected inside a micelle when immersed in a polar solvent).įrom a chemical perspective, surfactants solve the incompatibility between waterĪnd oil because they contain both polar and nonpolar properties, serving as a bridge between polar/aqueous and nonpolar/organic substances. In the case of water-oil interface, adding a surfactant will lower the energy barrier for breaking hydrogen bonds among water molecules, and allow more freedom for water molecules to interact with other types of molecules in a given system. Surfactants are molecules or molecular compounds that lower the surface tension at an interface (interfacial tension). How can we lower the energy barrier to prevent separation? The answer lies in the addition of emulsifying agents, also known as surfactants. However, there are many contexts that require the stabilization of oil and water mixtures. We might shake vigorously to mix oil and water together, but for the reasons mentioned above, the mixture will quickly separate into two distinct phases.


 0 kommentar(er)
0 kommentar(er)
